Gold has historically stood as the ultimate physical constant. Its refusal to oxidize or interact with the elements around it earned it the designation of a noble metal. This chemical isolationism made gold the ideal candidate for currency and high-end electronics, because it remains unchanged by time or atmosphere. However, this reputation for being unreactive is a product of a specific terrestrial environment rather than an absolute law of physics.
Behind the facade of nobility lies an atomic structure that can be manipulated if the external forces are sufficiently extreme. For decades, theorists suggested that the rules of the periodic table might buckle under the conditions found at the center of a planet. If gold could be forced to bond with the most basic element in the universe, the current understanding of material stability would require a significant revision.
The tension between gold’s stubborn nature and the crushing gravity of gas giants has reached a resolution. In January 2026, researchers bridged this chemical divide by creating a compound that was previously considered impossible under laboratory conditions. This shift does not just change the chemistry of a single metal; it recalibrates the expectations for every element in the solar system.
Synthesizing Gold Hydride at the Megabar Threshold
Records from the experimental campaign, first reported in Angewandte Chemie in late 2025, indicate that the synthesis of gold hydride occurred at pressures exceeding 110 gigapascals. This achievement utilized a diamond anvil cell to compress gold samples within a hydrogen-rich chamber. Data shows that under these intensities, the gold lattice expands to accommodate hydrogen atoms, creating a stable chemical bond. This process requires forces equivalent to over one million times the pressure of Earth’s atmosphere.
The transformation was verified using the European XFEL, where ultrashort X-ray pulses captured the atomic rearrangement in real-time. These pulses, measured in femtoseconds, act as a high-speed camera for chemical reactions. Based on the diffraction patterns collected, the gold atoms shifted into a new configuration to share electrons with the surrounding hydrogen. This specific molecular structure represents the first time a noble metal has been synthesized into a stable hydride under these conditions.
The electronic structure of gold normally prevents such interactions due to the stability of its outer electron shells. However, the application of a megabar of pressure physically overlaps these shells with those of the hydrogen atoms. Results show that the gold atoms lose their “noble” resistance and begin to function as a reactive transition metal. This behavior suggests that the chemical identity of an element is a variable dictated by its gravitational environment.
The Role of X-Ray Free-Electron Lasers in High-Pressure Analysis
Capturing the formation of gold hydride requires a level of visual clarity that traditional microscopes cannot provide. The use of X-ray Free-Electron Lasers (XFEL) has changed the methodology of high-pressure chemistry by allowing scientists to see through the dense diamond anvils. These lasers produce light that is billions of times brighter than conventional synchrotron radiation. This intensity is required because the sample being studied is smaller than a grain of dust and is obscured by the thick diamond windows of the pressure chamber.
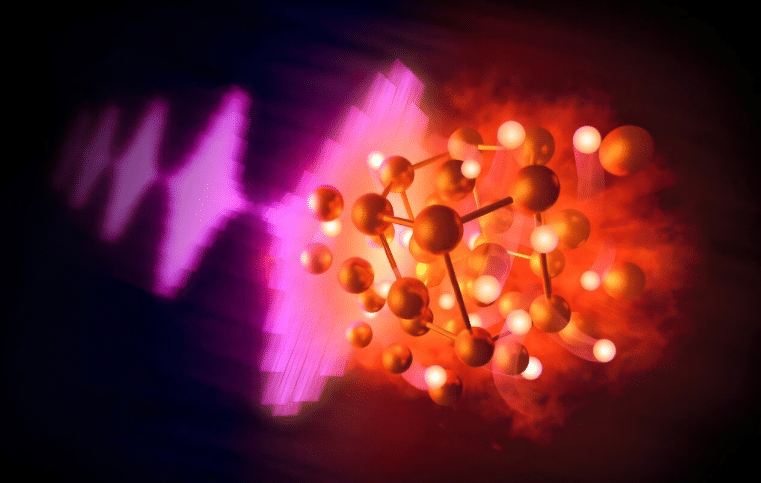
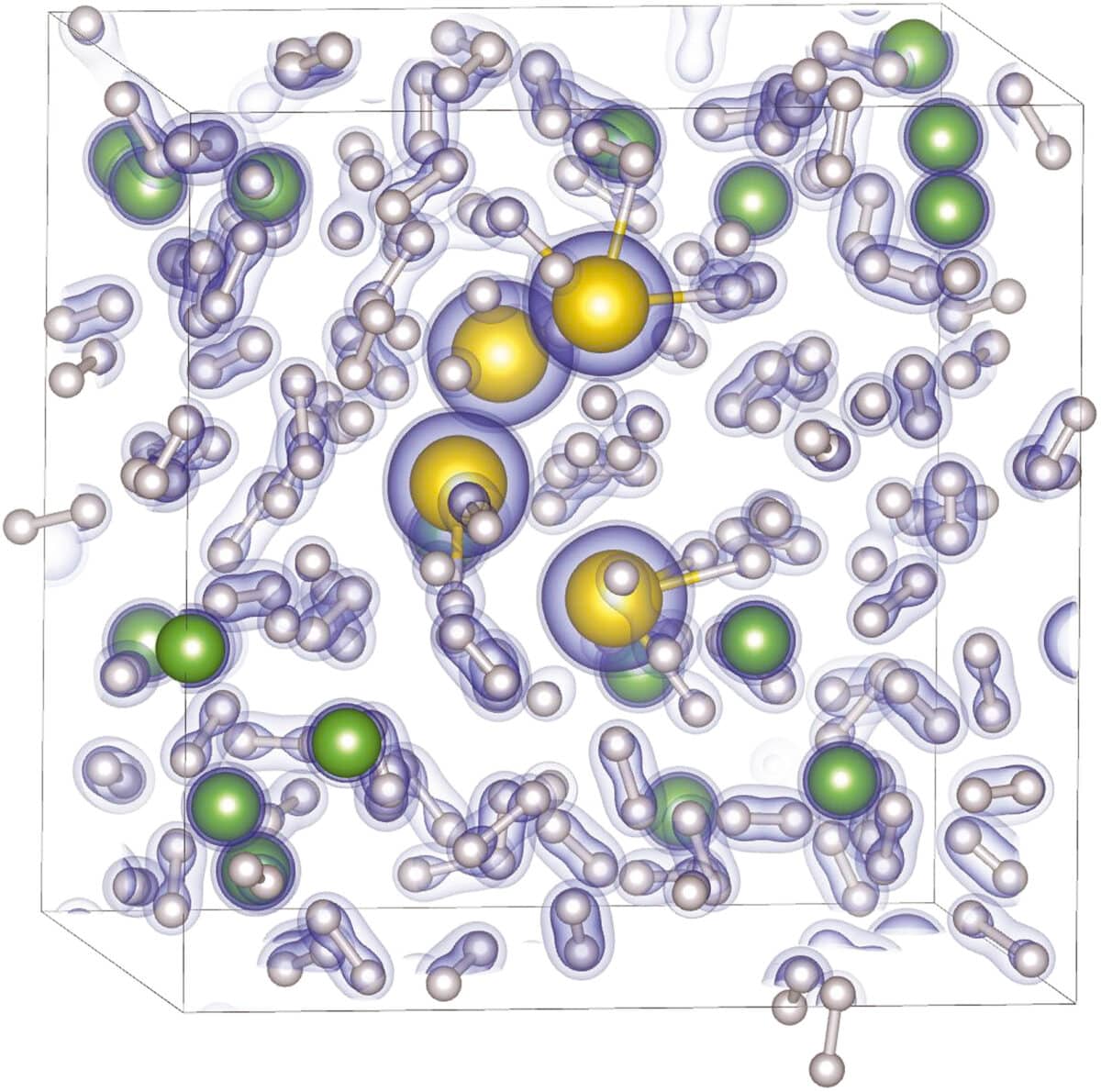
Technicians at the SLAC National Accelerator Laboratory utilize a method known as X-ray diffraction to identify the atomic arrangement of the hydride. When the X-ray beam hits the gold-hydrogen lattice, the light scatters in a predictable pattern. By analyzing the angles and intensities of this scattered light, researchers can reconstruct the exact positions of the atoms. In the case of gold hydride, the diffraction data showed a significant increase in the volume of the unit cell, a clear indicator that hydrogen had been integrated into the gold structure.
The operational reality of these experiments is exceptionally demanding. The diamonds used in the anvil cells must be flawless to withstand the internal stress; even a microscopic inclusion can cause the diamonds to shatter instantly under megabar pressures. Furthermore, hydrogen is difficult to contain at these levels. Because hydrogen atoms are the smallest in the universe, they tend to diffuse through the metal and the diamond itself. Success in January 2026 was facilitated by new tungsten-based sealing techniques that maintained the hydrogen pressure throughout the analysis.
Redefining Planetary Interior Models and Material Limits
The existence of gold hydride under extreme conditions has consequences for how astrophysicists model the interiors of gas giant planets. For decades, the cores of planets like Saturn and Neptune were thought to be composed of relatively simple mixtures of rock, ice, and metallic hydrogen. The discovery that noble metals can react with hydrogen suggests that the chemical gradients within these planets are far more complex. If gold can form hydrides, it is highly likely that other heavy elements also form stable compounds that alter the density and magnetic properties of planetary interiors.
The groundwork for this discovery was laid years prior in a study published by the American Geophysical Union in 2016. That research detailed the theoretical phase relations in the Au-H system and identified the specific temperatures where gold might lose its inert status. By 2025, advancements in laser-heating technology allowed scientists to reach the required thermal thresholds while maintaining megabar pressures. This synergy of pressure and heat is what finally triggered the metallization of the gold-hydrogen mixture.
These laboratory observations mirror the suspected chemical environments within the deep mantles of the outer planets. If gold and other dense elements can bond with hydrogen, they may be distributed throughout the hydrogen-rich layers rather than sequestered entirely in a solid core. This would influence the thermal conductivity and magnetic field generation of these planets. Current planetary models are being updated to include these metallic hydrides in their density calculations.
The Future of Synthetic Material Stability
The strategic implications for materials science involve the potential for superconductivity and high-energy-density storage. Researchers are now investigating whether the properties of interstitial hydrogen in gold can be replicated in more common metals to create new types of conductors. The ability to manipulate the electron sharing of “stubborn” elements opens the door to a new class of synthetic materials. These materials exist only at the edge of physical possibility, yet they provide the blueprint for future industrial applications.
Efforts are now focusing on the behavior of other group 11 elements under similar conditions. Preliminary data suggest that silver and platinum may follow a similar path toward reactivity at even higher pressures. The 2026 discovery confirms that the periodic table’s behavior is fundamentally tied to gravitational forces. Ultrafast imaging continues to serve as the primary tool for documenting these changes.
The compound remains a creature of the deep and exists only as long as the diamonds are pressing together. If the pressure is released, the hydrogen escapes the lattice and the gold returns to its original state. This limitation means that currently, gold hydride can only be studied in situ using high-energy diagnostic tools. The experiment concluded with the measurement of a 15 percent increase in the gold unit cell volume during the hydride formation.
First Appeared on
Source link





Leave feedback about this