Compass Pathways on Tuesday disclosed results from two Phase 3 studies that support a potential approval of its psilocybin treatment for severe depression, but more detailed data are needed to determine how beneficial the drug would be for patients.
In both trials, patients who received the company’s psychedelic medicine saw greater improvements on a measure of depression than the control group, Compass said in a press release. Its drug, called COMP360, could be the first psilocybin product on the market and the second psychedelic approved after Johnson & Johnson’s Spravato, a ketamine derivative.
Taken together, the data “probably meets the bar for approval. It doesn’t shout out to you that this is miraculous,” said Jerry Rosenbaum, director of Massachusetts General Hospital’s Center for Neuroscience of Psychedelics, who was not involved with the study.

This article is exclusive to STAT+ subscribers
Unlock this article — plus daily coverage and analysis of the biotech sector — by subscribing to STAT+.
Already have an account? Log in
First Appeared on
Source link
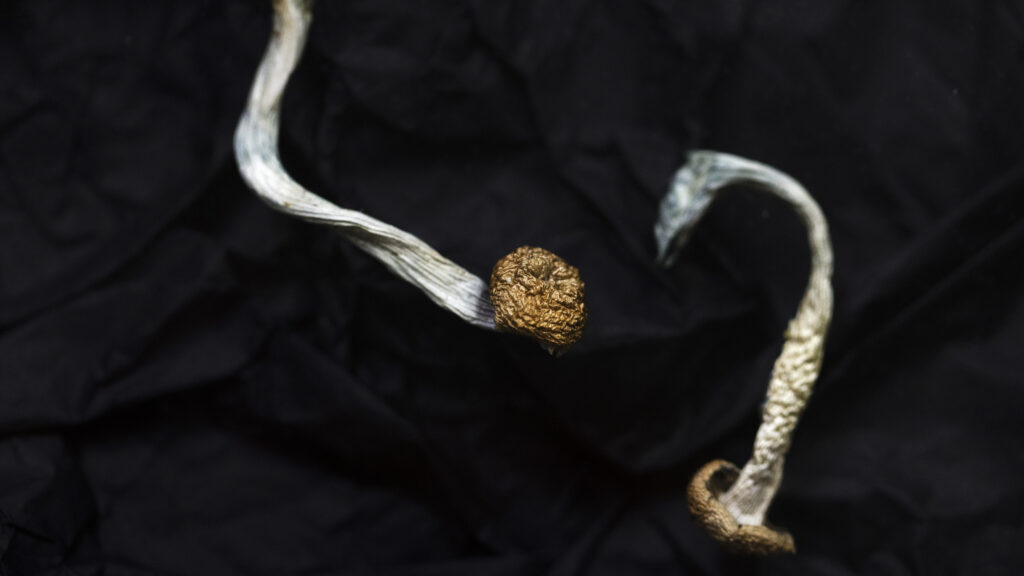






Leave feedback about this